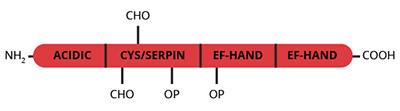
Osteonectin is an acidic, noncollagenous glycoprotein (Mr=29,000) originally isolated from fetal and adult bovine bone matrix (1-5). In vitro bovine bone osteonectin binds type I collagen, calcium (Kd=3×10-7 M) and hydroxyapatite (Kd=8×10-8 M) and has been shown to be a potent inhibitor of hydroxyapatite seeded crystal growth (6). In this context it has been suggested that osteonectin may play an important role in the regulation of bone metabolism by binding hydroxyapatite to collagen. Recently, proteins homologous to osteonectin have been identified in a number of cell types, most of which are associated with extracellular matrix production (7). The amino acid sequence (from cDNA sequences) of one of these proteins, human placental SPARC is identical to human bone osteonectin.
Osteonectin has also been identified as an alpha granule component of human platelets and is secreted during activation. A small portion of the secreted osteonectin is expressed on the platelet cell surface in an activation dependent manner (8). Purified platelet osteonectin is a single chain molecule which exhibits a slightly larger apparent molecular weight than that of osteonectin derived from bone (8). The NH2-terminal sequences of platelet and bone-derived osteonectin are identical, but the two proteins differ with regard to the extent ot glycosylation (8-10).
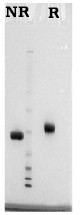
Human platelet osteonectin is isolated from thrombin activated platelets as described by Kelm, et al. (8). Bovine bone osteonectin is isolated from demineralized bone by the method of Romberg et al. (2). Both proteins are supplied in 0.02 M Tris, 0.15 M NaCl, pH 7.4, and should be stored at -80oC. Purity is judged by SDS-PAGE analysis.