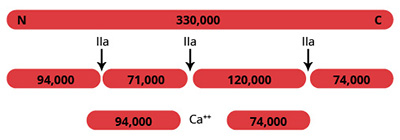
Factor V is a large, single chain, plasma glycoprotein which is an essential component in the blood coagulation cascade (1). During coagulation, the procofactor, factor V, is converted to the active cofactor, factor Va, via limited proteolysis by the serine protease alpha-thrombin (illustrated above), and less efficiently by factor Xa. The active cofactor is composed of an NH2-terminal derived heavy chain (Mr=94,000) and a COOH-terminal derived light chain (Mr=74,000) which remain non-covalently associated in the presence of calcium ions. Factor Va serves as a cofactor for the serine protease factor Xa. Factor Va and Xa assemble on a phospholipid surface in a non-covalent and calcium ion-dependent manner, to form the prothrombinase complex. The prothrombinase complex is responsible for the rapid conversion of the zymogen prothrombin to the active serine protease, alpha-thrombin. Assembly of the prothrombinase complex increases the rate at which prothrombin is converted to thrombin by nearly 300,000-fold relative to the rate with factor Xa alone. Down regulation of the prothrombinase complex is accomplished partly through the inactivation of factor Va by activated protein C.
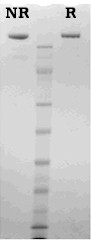
Human factor V is prepared from fresh frozen human plasma using immunoaffinity chromatography as described by Katzmann and coworkers (2). Bovine factor V is prepared from fresh bovine plasma using conventional chromatographic techniques as described by Nesheim and coworkers (3). Purified factor V is supplied in 50% glycerol (vol/vol), and should be stored at -20°C. Purity is determined by SDS-PAGE analysis and activity is measured in a factor V clotting assay.