Human protein Z (PZ) is a single chain, vitamin K-dependant plasma protein (1,2). Analogous with the majority of the coagulation proteins, protein Z is synthesized in the liver. The mature protein contains 360 amino acids (4). Based on amino acid sequence homology the domain structure is similar to that of other vitamin K-dependant zymogens which include; factor VII, factor IX, factor X, and protein C (3,4). The N-terminal region contains a carboxyglutamic acid (Gla) domain important in its phospholipid membrane binding ability (5). Following the N-terminal Gla domain are two EGF domains and a region which connects to a catalytic-like domain (3,4). The C-terminal region has been shown to lack the “typical” serine protease activation site as well as the His and Ser residues from the catalytic triad (3,4). Protease activity has not been detected in either the full-length protein or cleavage products of protein Z (2). Functionally protein Z has been shown to be a direct requirement for the binding of thrombin to endothelial phospholipids (6,7). Protein Z also serves as a cofactor for the inhibition of coagulation factor Xa by a plasma serpin called protein Z-dependant protease inhibitor (ZPI) (8). Inhibition is dependant upon complex formation between factor Xa-PZ-ZPI on the phospholipid surface (8).
The physiological function of protein Z is still rather ill defined. As is the case with other coagulation proteins and inhibitors, protein Z is consumed during disseminated intravascular coagulation (DIC) (9). Furthermore, patients diagnosed with a protein Z deficiency present with abnormal bleeding diathesis during and after surgical events (10). These findings provide direct evidence as to the importance of protein Z in blood coagulation.
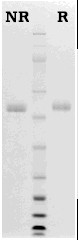
Human protein Z is prepared from fresh frozen plasma similar to the procedure described by Broze and Miletich (2). The purified protein Z is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20oC. Purity is assessed by SDS-PAGE analysis.