β-thromboglobulin (b-TG), is a low molecular weight, heparin-binding, platelet-derived protein (1). It is similar to platelet factor-4 (PF-4) in that it is localized within the platelet alpha-granule at levels reported to range from 8.1-24.2 µg per 109 platelets (2,3). The relative concentration of β-TG in platelets exceeds that of plasma by 260,000-fold (4) making β-TG a convenient marker of platelet activation. Structurally, β-TG is analogous to PF-4 in that, in its native state, β-TG is a tetramer (1) consisting of four identical 8800 molecular weight peptide chains (5). In contrast to PF-4, β-TG exhibits a lower affinity for heparin and also exists as a larger molecular weight species known as “low affinity PF-4” (LAPF-4) (2). β-TG is derived from the proteolytic removal of four NH2-terminal amino acid residues from a LAPF-4 (6,7). Immunological screening of partially fractionated supernatant from activated platelets revealed a highly basic form of β-TG distinct from LAPF-4 (7). This basic β-TG species, termed platelet basic protein (PBP), was subsequently isolated (8) and later concluded from immunological, peptide sequencing, and proteolytic processing studies to be a higher molecular weight precursor form of both LAPF-4 and β-TG (9,10).
The physiological function of β-TG is not known. While early studies suggested that the precursor forms of β-TG were mitogenic for mouse fibroblasts (8,11), it was later concluded that this activity was due to growth factor contamination (10). β-TG has also been reported to inhibit prostacyclin-I2 production by endothelial cells (12), however, the relevance of this effect has been called into question (13,14). The chemotactic activity of platelet alpha-granule proteins for human fibroblasts has been attributed to both PF-4 and β-TG (15).
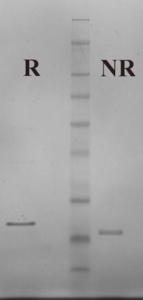
Human β-TG is prepared from the supernatant of activated platelets by heparin-agarose affinity chromatography and gel filtration (1,2). The purified protein is supplied in 25 mM Hepes, 150 mM NaCl pH 7.4 and should be stored at -80°C. Purity is assessed by SDS-PAGE analysis.