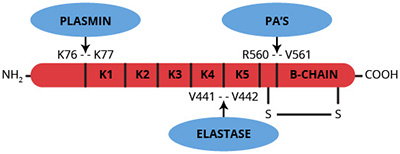
Plasminogen (whether Glu-1, Lys-77 or Val-442) is converted to the active serine protease plasmin by hydrolysis of the Arg560-Val561 peptide bond yielding an NH2-terminal heavy (A) chain and a COOH-terminal light (B) chain linked by 2 disulfide bonds (1-3). This conversion is catalyzed by a variety of physiological and pathological activators, including urinary type plasminogen activators, tissue type plasminogen activators, streptokinase, staphylokinase, kallikrein, factors IXa and XIIa. The COOH-terminal derived light chain (Mr=26,000) contains the catalytic triad (His42, Asp85 and Ser180) as well as the streptokinase binding site. The NH2-terminal derived heavy chain ranges in molecular weight from 63,000 to 12,000 depending on the type of plasminogen from which it originated. In the absence of inhibitors, plasmin cleaves the amino-terminal Glu1 to Lys76 peptide from plasmin (plasminogen) to yield Lys-plasmin, which has a greater affinity for fibrin than the Glu form. The heavy chain of Lys-plasminogen contains 5 triple loop disulfide bridged regions of internal sequence homology known as kringles. Kringles 1-4 contain the ω-aminocarboxylic acid and fibrin binding sites.
Plasmin is a serine protease with broad specificity which, in addition to cleavage of fibrin, is capable of activation and/or degradation of compounds of the coagulation, kinin generation and complement systems. Although plasmin can be inhibited by a number of plasma protease inhibitors in vitro, regulation of plasmin in vivo is thought to occur mainly through its interaction with α2-antiplasmin, and to a lesser extent, α2-macroglobulin.
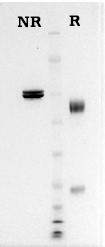
Human Lys-plasmin is prepared from homogeneous Glu-plasminogen using urokinase, as described by Robbins et al. (3). Plasmin is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20°C. Activity is measured by chromogenic substrate assay and purity is judged by SDS gel electrophoresis.